British citizens’ deaths in Spain have been linked to a banned painkiller, leading to concerns that I had cancer.
A group of patients, consisting of multiple British individuals, has initiated a lawsuit against the Spanish government for allegedly neglecting to protect citizens from the potentially deadly side effects of a commonly used pain reliever. This medication has been linked to numerous severe illnesses and fatalities.
The drug metamizole, commonly sold in Spain under the brand name Nolotil, is banned in several countries, including Britain, the US, India and Australia. It can cause a condition known as agranulocytosis, which reduces white blood cells, increasing the risk of potentially fatal infection.
The ADAF reports that negative responses to medications have resulted in sepsis, organ failure, and amputations. They have documented approximately 350 potential instances of agranulocytosis from 1996 to 2023, including 170 individuals from Britain who either reside in Spain or were vacationing there.
The ADAF is investigating over 40 deaths that could potentially be linked to the drug, either as the direct cause or a contributing factor. According to the patients group, case reports and a study from 2009 indicate that the British population may be more vulnerable to the drug’s adverse effects, but this has not been verified by an independent scientific study.
The organization is calling for an inquiry into the medication and stricter regulations. They submitted their request on November 14th in the Madrid national court. Cristina García del Campo, the founder of the group, stated: “This medication has caused immense harm to individuals and should be removed from circulation. One woman who took three tablets had to have part of her feet amputated and lost multiple fingers. Even if it doesn’t result in death, sepsis permanently changes the body.”
The first commercial production of Metamizole occurred in Germany in 1922 and it was readily accessible globally. However, after discovering its potential to cause agranulocytosis, it was discontinued in approximately 30 countries. Despite this, it remains easily accessible throughout the EU.

Research has revealed a significant difference in the predicted occurrence of agranulocytosis when taking the drug, ranging from approximately 1 in 2,000 to less than 1.1 per million users. A report by the European Medicines Agency in December 2018 proposed that the possibility of causing agranulocytosis may be linked to specific genetic traits in certain populations.
García del Campo, a translator from Jávea in Alicante, began looking into the matter when a client of hers, who was Irish, became extremely sick with various infections throughout his body. He was hospitalized in Dénia and passed away on November 18, 2017 due to sepsis and failure of multiple organs.
She recounted, “I was the final individual to be with him and I grasped his hand. Throughout my time with him, I continuously questioned, ‘Why is this occurring? How could one’s health suddenly deteriorate from being well to contracting such a severe infection?'”
In December 2017, she organized and reviewed multiple case files and medical records related to six cases she had heard about in her local area. As she examined them, she realized that all of the individuals had taken metamizole and were experiencing troubling conditions such as agranulocytosis and sepsis.
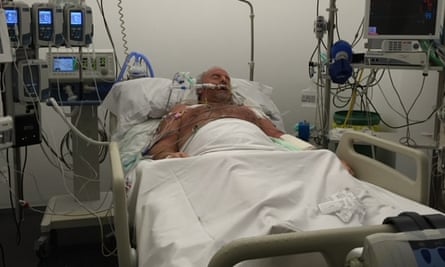
Last week, Paddy Clancy, an 80-year-old British expatriate living in Jávea, shared his experience of being treated by García del Campo. He nearly lost his life after being administered metamizole following a shoulder surgery in September 2017. His condition deteriorated to the point where doctors had to induce a coma to increase his chances of fighting the infection.
Clancy reported that his spouse was informed that his kidneys were failing and his organs were ceasing to function. They were concerned he may not survive the weekend. The doctor also revealed that the white blood cells responsible for fighting infection in his body were dangerously low.
After 39 days, Clancy emerged from his coma. He had lost 22kg and was unable to stand, but has since made a gradual recovery. His medical records confirm the condition that almost took his life: “agranulocytosis linked to metamizole.”

García del Campo had discovered numerous instances that were similar. In April 2016, William Smyth, a 66-year-old Irish tourist, passed away due to multi-organ failure after being prescribed Nolotil for shoulder pain. Mary Ward, a 59-year-old resident of Spain, also died in March 2006 after being given Nolotil following a surgery in Marbella and developing agranulocytosis and complications. In a separate case, a woman in her 60s had to undergo amputations after taking the medication and experiencing sepsis.
According to reports, agranulocytosis is a rare reaction to metamizole, and García del Campo received numerous reports about it. These cases suggested that the British and Irish populations were more susceptible. In April 2009, a study at Hospital Costa del Sol in Marbella examined 13 cases of agranulocytosis related to dipyrone (another name for metamizole), with five of the cases involving Britons. The study concluded that dipyrone should be avoided due to its higher occurrence of agranulocytosis in the British population.
The ADAF presented evidence in their case from a health official in the region. The official stated that a study conducted in five health departments in Spain showed a surprisingly high susceptibility to metamizole in the British population, with a ratio of 80 to 120 times higher than the Spanish population. However, this report has not been published and there is currently no strong epidemiological evidence to support the belief that British or Irish individuals may be more prone to experiencing side effects from the drug.
In April 2018, Lorna Vincent, a 75-year-old resident of Spain, underwent surgery in Benidorm to fix a small hole in her bowel. According to her daughter, Kim Glasby, who is 59 and from Brixham, Devon, the surgery initially seemed to be successful and her mother was prescribed metamizole for pain relief. However, she later became seriously ill. Glasby recalls being informed by the surgeon that her mother’s white cell count was low and she was not responding to pain medication. The medical team was unsure of how to proceed.

Vincent passed away on April 18th after experiencing multi-organ failure. Based on other similar cases, Glasby suspects that a reaction to metamizole was the cause of death and is currently working to obtain her mother’s complete medical records.
In October of 2018, just under a year since García del Campo began her campaign, the Spanish Agency for Medicines and Health Products (AEMPS) released updated guidelines for metamizole. The guidelines advised against its use in tourists (referred to as the “floating population”) and recommended that patients be informed of symptoms of agranulocytosis.
According to García del Campo, the recently implemented guidelines have been frequently disregarded. She pointed out that patients are not being adequately informed of the potential dangers and that the medication can be obtained without a necessary prescription.
Carla Cardwell, age 41, was born in the UK but currently resides in Gibraltar. In December 2019, she delivered her son, Caiden, via caesarean section in the town of La Línea, just across the Spanish border. She was prescribed metamizole for pain relief.
In January 2020, she became very sick and visited a nearby A&E unit in Gibraltar. She was informed that she had no white blood cells and was suspected to have cancer. However, the consultants were puzzled as her blood results were indicative of cancer but she did not actually have it.
A doctor with extensive experience examined her situation and inquired about her recent use of metamizole. She confirmed she had taken it and was subsequently diagnosed with agranulocytosis. The doctor recommended that she receive injections of granulocyte colony-stimulating factor (G-CSF) to help regenerate her bone marrow.
She recounted: “My pancreas, liver, and bowel were all affected by infection. The injections had to be transported to the hospital and were the most difficult part. I could even sense the pain in my eyes. I will always be thankful to the consultant who saved me.” She received therapy for post-traumatic stress following her experience. Her medical documents indicate she experienced agranulocytosis, with metamizole as the suspected trigger.
The ADAF has taken legal action against the Spanish Ministry of Health and the AEMPS, stating that the drug is being distributed to patients without appropriate supervision. The organization is calling for a prohibition on providing the drug to citizens from countries where metamizole has been banned, as well as a reevaluation of the potential risks associated with agranulocytosis. Additionally, they are requesting a revision of the drug’s information sheet.
The attorney for the ADAF, Francisco Almodóvar, stated that they have received accounts from British individuals sharing their experiences. They are able to back up this evidence with medical records, highlighting the importance of this issue in terms of public health.
Boehringer Ingelheim, the manufacturer of Nolotil, stated that it is a medication that requires a prescription. The active ingredient, metamizole, has been utilized by patients for nearly a century and has a well-documented record of safety.
“Agranulocytosis is described as a very rare frequency adverse reaction in the current prescribing information. It is a known adverse reaction for decades and the available scientific information has confirmed the well known safety profile of metamizole.
The current information about Nolotil addresses the potential side effect of agranulocytosis. The prescribing information adequately covers the known risks of using Nolotil. We appreciate any additional information that can help us improve the balance of benefits and risks and ensure safe use of our medication.
The AEMPS directed the Observer to the Spanish health ministry, who did not reply to a comment request.
Source: theguardian.com


