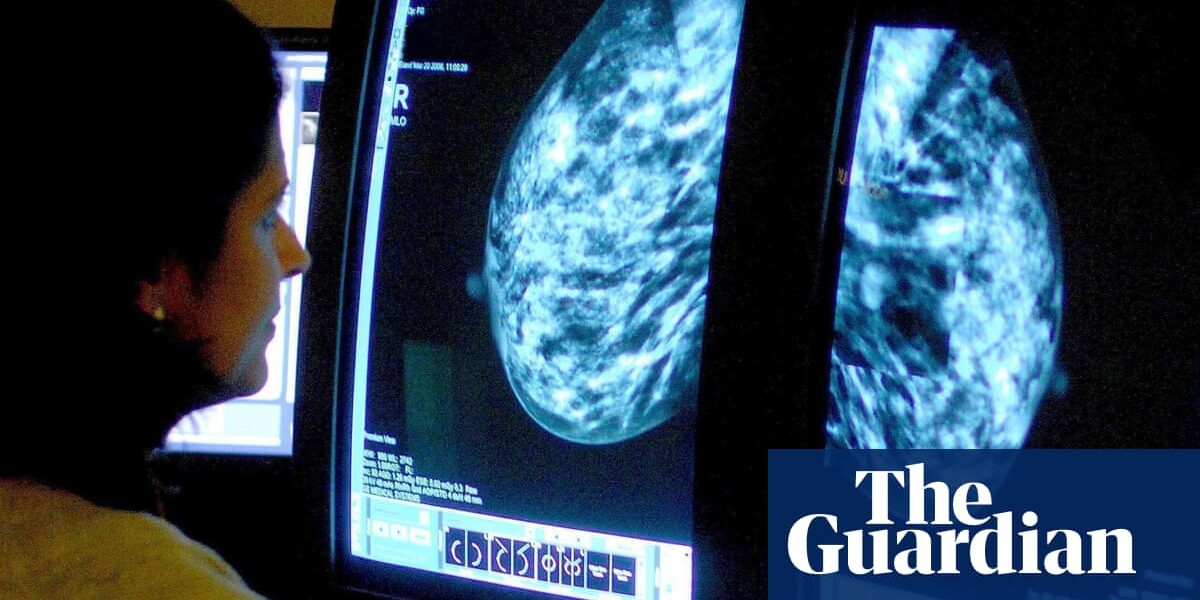
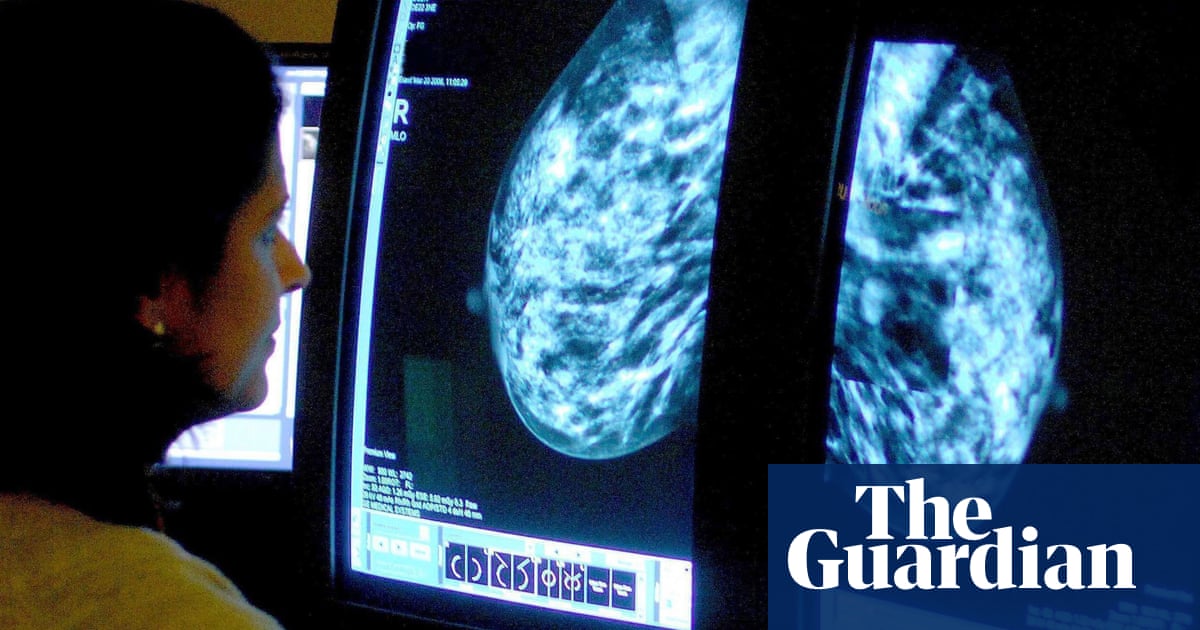
A potential treatment for breast cancer could benefit a significantly higher number of women than originally estimated.
Research indicates that a significantly larger number of female individuals with breast cancer may be able to utilize an extremely successful immunotherapy medication.
The medication Pembrolizumab, also known as Keytruda, inhibits a particular protein found on certain immune cells, causing them to locate and eliminate cancer cells.
Triple-negative breast cancer, a particularly aggressive type of cancer that makes up 15% of all cases, may now be eligible for treatment in England based on recent results from a worldwide study.
The results will be showcased at the European Breast Cancer Conference in Milan this Wednesday.
Researchers have found that a combination of pembrolizumab and chemotherapy may be beneficial in treating a prevalent type of breast cancer, irrespective of the patient’s age or menopause status, before and after surgery.
The study tested a drug on women who have ER-positive and HER2-negative early-stage breast cancer with a high risk of recurrence or metastasis.
Cancer Research UK reports that approximately 80 out of every 100 breast cancer diagnoses are classified as ER positive.
The international Keynote-756 study has been ongoing for eight years and involves 1,278 participants diagnosed with invasive ductal carcinoma (IDC), which indicates that the cancer has begun to spread from the milk ducts to the neighboring breast tissue.
The study involved administering either pembrolizumab and chemotherapy before and after surgery, or a placebo, to patients. The researchers evaluated the presence of cancer indicators in tissue samples, referred to as pathological complete response (PCR) rate.
According to Prof Javier Cortés, the head of the International Breast Cancer Centre in Barcelona, Spain, there was a notable rise in PCR rate for those who received pembrolizumab treatment.
Approximately 24.3% of patients did not have any cancer cells present in their breast or lymph nodes, while only 15.6% of patients treated with a placebo had the same outcome.
Dr. Simon Vincent, the director of research, support, and advocacy at UK’s Breast Cancer Now charity, expressed his enthusiasm for the findings of this study. According to him, the combination of pembrolizumab and chemotherapy before and after surgery could potentially be more successful in eradicating cancer cells in women with the most prevalent type of breast cancer, ER-positive HER2-negative, regardless of their age or menopausal status.
The test showed that pembrolizumab resulted in a higher number of patients with no visible cancer cells in their breast or lymph nodes at the end of their treatment. However, more studies are required to determine if this leads to better survival rates and a decreased chance of the cancer recurring.
”
“With a monthly death toll of 1,000 due to breast cancer in the UK, there is an urgent need for innovative and successful approaches to treating this condition.”
“We anticipate that pembrolizumab, currently utilized in the treatment of triple negative breast cancer, will undergo review by the MHRA for licensure and evaluation by NICE at the earliest opportunity. This would grant patients with ER-positive HER2-negative breast cancer, who could potentially benefit from this drug, access to it through the NHS.”
The conference will also hear how researchers have developed a genetic test that can identify how patients with triple negative early-stage breast cancer will respond to immunotherapy drugs. It means patients who are unlikely to respond to these drugs can avoid the adverse side-effects associated with them and can be treated with other therapies.
Source: theguardian.com